QUANTIFY RESEARCH is a global partner to the pharmaceutical industry in close partnership with academia, patients, public institutions & clinical experts.
Real-world evidence & analytics
Quantify is the leading provider of RWE generated from the world-renouned Nordic data. Through our local presence, expert staff, institutional know-how and more than 10 years’ hands-on experience with the Nordic data, we offer an unparalleled ability to advise and execute Nordic RWE studies with local and global applications. Quantify has a successful track record of advising clients on securing Nordic ethical approval, data access, and optimizing analysis for commercial or regulatory purposes. We also offer RWE services in the EU, UK, and US.
Modelling, Access & Strategy
Quantify is a global provider of health economic evidence and a specialist provider of Nordic market access services including economic models, reimbursement dossiers, and strategic advice. Our experience and expertise ensures an optimized, streamlined market access process across the Nordics. For non-technical stakeholders, Quantify also develops value tools and visualisation dashboards to enhance communication. Our expert staff include ex-payers, ex-pharma, modelling experts, and experienced project managers.
Evidence review & synthesis
Quantify has long standing experience reviewing, interpreting and communicating evidence as part of targeted or systematic literature reviews (SLR). Our team ensures these activities are done systematically, with a focus on usability, to support knowledge management and commercialization activities. QUANTIFY is experienced in summarizing the results of this work through meta-analyses and narrative reviews. Our team consists of 50% PhDs and integrated AI solutions to provide our clients with the very best services.
Press
A great evening at Quantify´s summer party!
Last Thursday the Nordic Quantify Research team got together for our annual summer party at Södra Teatern.
Thanks to everyone who attended for a great evening filled with a scavenger hunt, dinner, and dancing! 💚


Mondays in Motion
📅 Each Monday, we lace up and get moving – it’s lunch run day!
👋 If you spot the team out on the move, give us a wave


📢Publication alert!
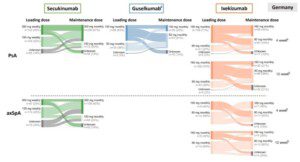
We’re excited to share our latest manuscript “Real-world Utilization of Biologic and targeted Synthetic Disease- Modifying Anti- Rheumatic Drugs in Psoriatic Arthritis and Axial Spondyloarthritis : Insights from Sweden and Germany”” now available online in 𝘈𝘥𝘷𝘢𝘯𝘤𝘦𝘴 𝘪𝘯 𝘛𝘩𝘦𝘳𝘢𝘱𝘺, co‑authored by Quantify’s Alexandra Cooper and alumni Alexander Rieem Dun and Christoph Abé.
Coordinated and executed by […]
How can we make innovative pricing models actually work?
An important part of the answer lies in real-world data you can trust. With over 300 register-based projects across the Nordics, we’ve seen firsthand how high-quality patient-level data enables:
✅ Follow-up on individual treatment outcomes
✅ Evaluation of long-term survival and disease activity
✅ Assessing the societal impact through sickness absence and costs
✅ Dynamic dashboards tailored for regions and indications
As healthcare systems evolve toward more sophisticated agreements including payment-over-time models or pricing […]

