Real-World Evidence & Analytics
QUANTIFY is the leading provider of Nordic real-world evidence (RWE) with vast documented experience in high-impact RWD studies. The QUANTIFY team possess expertise in data access, analysis, and interpretation with an unparalleled understanding of Nordic data in Denmark, Finland, Norway, and Sweden. We can access linked, patient-level data across the Nordics spanning primary care and hospital visits, drug prescriptions, sociodemographic, economics, labs, electronic health records (EHRs), patient-reported outcome measures (PROMs) and others to produce innovative, impactful research.
Our team understands the increasing role RWE plays in clinical, economic and regulatory decision-making, throughout a product lifecycle, from the pre clinical research phase to the post-market authorisation and commercialisation phase. We provide research- and regulatory-grade RWE with many applications, across many disease areas with a focus on meeting the needs of various stakeholders. In order to meet this high standard, QUANTIFY combines a deep understanding of data, methods and clinical context to provide meaningful insights and robust results.
In order to streamline cross-Nordic RWE analyses, we have developed a common data model (CDM) called Qniform, to optimize the research process and interpretability of results.
QUANTIFY recognizes that while Nordic data is unparalleled in many ways, manufactures also need to develop evidence in other geographies. That’s why we work with a network of talented partner organisations to offer a wide range of coordinated RWE across the EU, US, and UK.
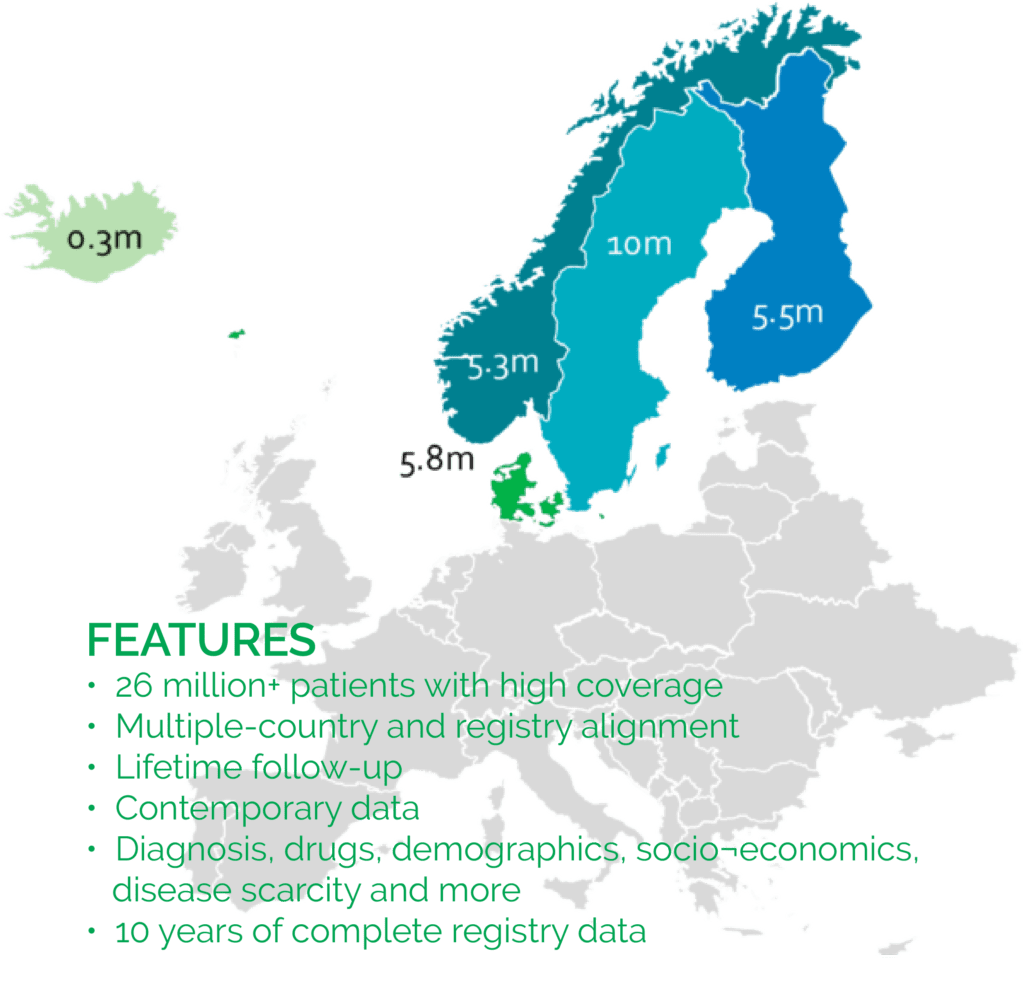
NORDIC REGISTRIES
Quantify is a top provider of Nordic register studies, having executed more than 200 projects that produced over 100 peer-reviewed publications in numerous therapeutic areas. Nordic data are population-based, secondary data assets globally lauded for their quality, detail and long follow-up. Given the high coverage rates and ability to link many types of patient-level data, these assets are often superior to alternative data sources in larger markets (e.g. Germany and the UK) for demonstrating value and unmet need.
Nordic administrative data can be linked to any of the 100+ disease-specific registers in Sweden containing clinical data and patient-reported outcomes in addition to biobank assets. Our partners increasingly rely on Quantify’s expertise in accessing, analysing, and interpreting Nordic data to support research programs globally.
Example of data types that may be accessed for research:
Quick access to anonymised patient-level statistics for feasibility or pilot studies
To determine whether a particular question can be answered using Nordic registries, Quantify can access key data in a matter of weeks.
- Access to data in as little as 2 weeks
- Can be used to validate and improve study design, check sample size, pinpoint subpopulations, test for outcomes etc.
- Data is anonymised through grouping (e.g. age bands) and/or masking (ICD-code to 3 digits)
- May be used to support HTA analyses
Track record
- 200+ observational data projects
- 100+ publications in peer reviewed journals
Methodological expertise
- Retrospective register/database analysis, chart reviews prospective data studies
- Epidemiology, comparative effectiveness, conjoint analysis, treatment patterns
- Frequentist, Bayesian, machine learning, and others
Data access expertise
- In-depth knowledge and understanding of content, strengths and limitations of national register data
- Experts in managing data access processes and extraction of research data in the Nordics
Network
- Quantify can access data via our extensive network that others can’t reach
- Connected with key opinion leaders, who can provide pivotal advice on clinical practice, study design and interpretation
SELECTED COLLABORATIONS
IMI ROADMAP
Project designed to provide a foundation for an integrated data environment and framework for real-world evidence research within Alzheimer’s disease.
IOF
NGO that functions as a global alliance of patient societies, research organizations, healthcare professionals and international companies working to promote bone, muscle and joint health.
IPECAD
IPECAD is a forum for the value demonstration of Alzheimer interventions. Quantify’s Anders Gustavsson sits in the steering committee and has recently co-developed an open-source cost-effectiveness model for AD.

