QUANTIFY RESEARCH is a global partner to the pharmaceutical industry in close partnership with academia, patients, public institutions & clinical experts.
Real-world evidence & analytics
Quantify is the leading provider of RWE generated from the world-renouned Nordic data. Through our local presence, expert staff, institutional know-how and more than 10 years’ hands-on experience with the Nordic data, we offer an unparalleled ability to advise and execute Nordic RWE studies with local and global applications. Quantify has a successful track record of advising clients on securing Nordic ethical approval, data access, and optimizing analysis for commercial or regulatory purposes. We also offer RWE services in the EU, UK, and US.
Modelling, Access & Strategy
Quantify is a global provider of health economic evidence and a specialist provider of Nordic market access services including economic models, reimbursement dossiers, and strategic advice. Our experience and expertise ensures an optimized, streamlined market access process across the Nordics. For non-technical stakeholders, Quantify also develops value tools and visualisation dashboards to enhance communication. Our expert staff include ex-payers, ex-pharma, modelling experts, and experienced project managers.
Evidence review & synthesis
Quantify has long standing experience reviewing, interpreting and communicating evidence as part of targeted or systematic literature reviews (SLR). Our team ensures these activities are done systematically, with a focus on usability, to support knowledge management and commercialization activities. QUANTIFY is experienced in summarizing the results of this work through meta-analyses and narrative reviews. Our team consists of 50% PhDs and integrated AI solutions to provide our clients with the very best services.
Press
ISPOREurope 2024 in Barcelona!

We are excited to announce our presence at this year’s ISPOREurope in Barcelona!
Over the course of the conference our colleagues look forward to getting some facetime with clients and colleagues and presenting research.
Get in touch to book a meeting or pop in to see us at Booth 622!
Quantify Research Finland has been accepted to Fingenious® Ecosystem!
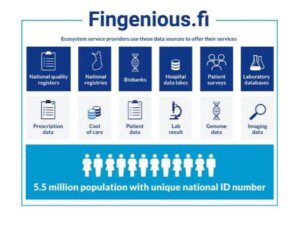
We’re happy to announce that Quantify Research Finland has been accepted to Fingenious® Ecosystem!
The Fingenious® Ecosystem is a collaboration network that includes various public, academic, and private actors in the field of health and biomedical research in Finland. The aim is to increase international research collaboration by providing a single […]
The Power of Social Listening in Real-World Evidence (RWE) Research
The landscape of healthcare research is rapidly evolving, and social media is emerging as a valuable resource for real-world evidence (RWE) studies.
In one of Quantify’s publications, 𝐒𝐨𝐜𝐢𝐚𝐥 𝐌𝐞𝐝𝐢𝐚 𝐋𝐢𝐬𝐭𝐞𝐧𝐢𝐧𝐠 𝐟𝐨𝐫 𝐑𝐞𝐚𝐥-𝐖𝐨𝐫𝐥𝐝 𝐄𝐯𝐢𝐝𝐞𝐧𝐜𝐞: 𝐀 𝐍𝐨𝐯𝐞𝐥 𝐀𝐩𝐩𝐫𝐨𝐚𝐜𝐡 𝐭𝐨 𝐇𝐞𝐚𝐫𝐢𝐧𝐠 𝐭𝐡𝐞 𝐏𝐚𝐭𝐢𝐞𝐧𝐭’𝐬 𝐕𝐨𝐢𝐜𝐞, the public sentiment towards vaccination was depicted by analyzing tweets.
This work shines a spotlight on how social media can revolutionize our approach to understanding patient experiences, treatment outcomes, and […]
World Mental Health Day October 10th 2024


To mark yesterday’s World Mental Health Day the team finished off their workday with a wonderfully relaxing (and sometimes challenging!) yoga session with Devon the Canadian Yogi. 🧘♀️
Our largest meeting room transformed into a haven of calm.
Very pertinent, since this year’s World mental Health Day theme is “Time to prioritize mental health in […]

