QUANTIFY RESEARCH is a global partner to the pharmaceutical industry in close partnership with academia, patients, public institutions & clinical experts.
Real-world evidence & analytics
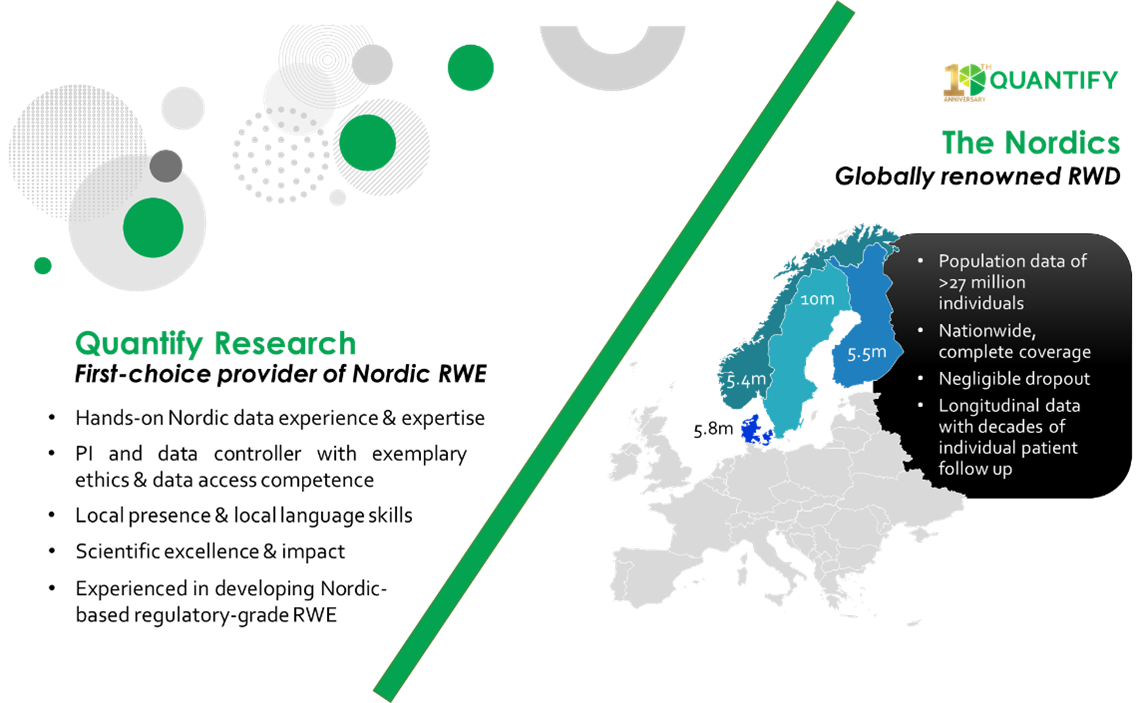
Quantify is the leading provider of RWE generated from the world-renouned Nordic data. Through our local presence, expert staff, institutional know-how and more than 10 years’ hands-on experience with the Nordic data, we offer an unparalleled ability to advise and execute Nordic RWE studies with local and global applications. Quantify has a successful track record of advising clients on securing Nordic ethical approval, data access, and optimizing analysis for commercial or regulatory purposes. We also offer RWE services in the EU, UK, and US.
Modelling, Access & Strategy
Quantify is a global provider of health economic evidence and a specialist provider of Nordic market access services including economic models, reimbursement dossiers, and strategic advice. Our experience and expertise ensures an optimized, streamlined market access process across the Nordics. For non-technical stakeholders, Quantify also develops value tools and visualisation dashboards to enhance communication. Our expert staff include ex-payers, ex-pharma, modelling experts, and experienced project managers.
Evidence review & synthesis
Quantify has long standing experience reviewing, interpreting and communicating evidence as part of targeted or systematic literature reviews (SLR). Our team ensures these activities are done systematically, with a focus on usability, to support knowledge management and commercialization activities. QUANTIFY is experienced in summarizing the results of this work through meta-analyses and narrative reviews. Our team consists of 50% PhDs and integrated AI solutions to provide our clients with the very best services.
Press
The increasing importance of real-world data
Earlier this week the U.S. Food and Drug Administration (FDA) granted accelerated approval to Vijoice® (alpelisib) based primarily on real-world evidence (RWE). The decision reflects the increasing importance of real-world data (RWD) for regulatory decision making, alongside recent guidance from the FDA and EMA.
As the leading provider of Nordic RWE studies, with more than 200 executed projects involving RWD, […]
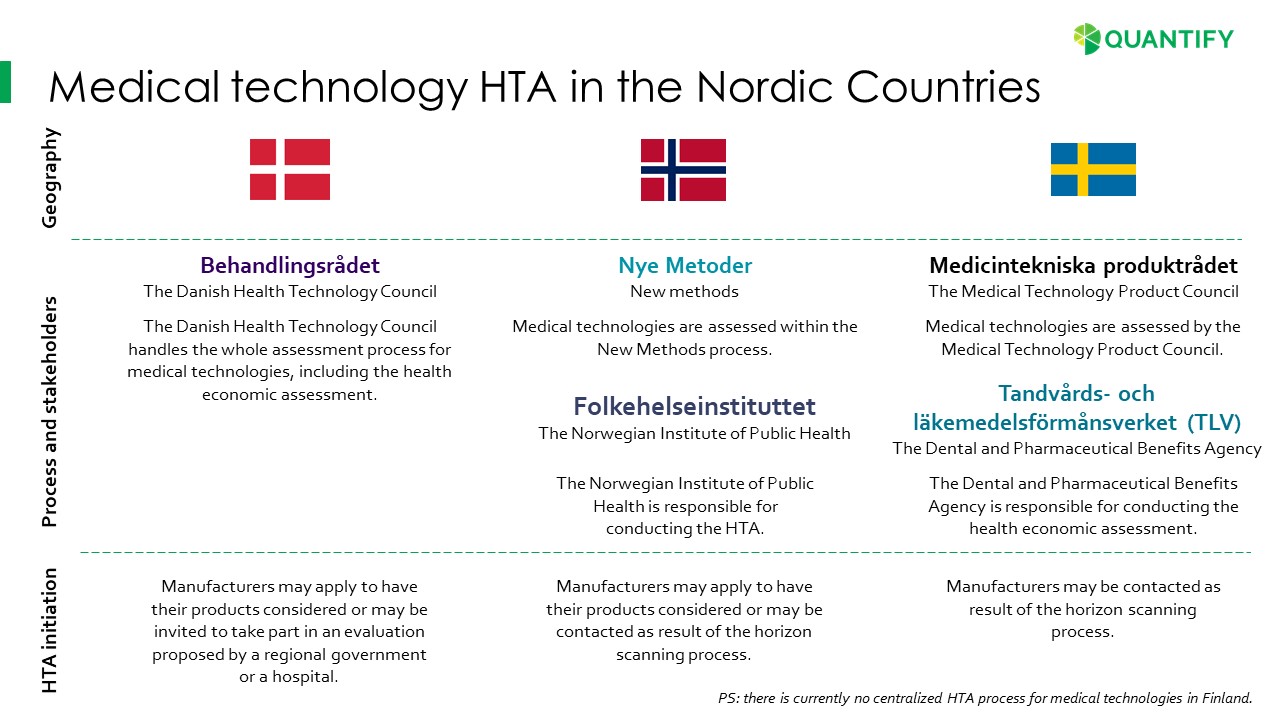
Confused with the new processes for medical devices HTA in the Nordics?
Douglas Knutsson presenter at SHEA conference in Gothenburg
Are you attending the 10th annual national conference of the Swedish Health Economics Association (SHEA) in Gothenburg this week? In that case we really hope that you were able to listen in on Quantify’s Douglas Knutsson yesterday when he presented an excerpt from our collaboration with Lif – de forskande läkemedelsföretagen.
#healteconomics #marketaccess #reimbursement #HEOR

Philip Gavuzzi joins Quantify!

Today we are happy to introduce Philip Gavuzzi who joined the Stockholm team as a Research Analyst last week. Philip has a background in economic analysis and hold a master’s in economics from Linköping University. He has previously worked within the financial sector as an AML-analyst but is now looking forward to exploring new areas at Quantify.
[…]

