QUANTIFY RESEARCH is a global partner to the pharmaceutical industry in close partnership with academia, patients, public institutions & clinical experts.
Real-world evidence & analytics
Quantify is the leading provider of RWE generated from the world-renouned Nordic data. Through our local presence, expert staff, institutional know-how and more than 10 years’ hands-on experience with the Nordic data, we offer an unparalleled ability to advise and execute Nordic RWE studies with local and global applications. Quantify has a successful track record of advising clients on securing Nordic ethical approval, data access, and optimizing analysis for commercial or regulatory purposes. We also offer RWE services in the EU, UK, and US.
Modelling, Access & Strategy
Quantify is a global provider of health economic evidence and a specialist provider of Nordic market access services including economic models, reimbursement dossiers, and strategic advice. Our experience and expertise ensures an optimized, streamlined market access process across the Nordics. For non-technical stakeholders, Quantify also develops value tools and visualisation dashboards to enhance communication. Our expert staff include ex-payers, ex-pharma, modelling experts, and experienced project managers.
Evidence review & synthesis
Quantify has long standing experience reviewing, interpreting and communicating evidence as part of targeted or systematic literature reviews (SLR). Our team ensures these activities are done systematically, with a focus on usability, to support knowledge management and commercialization activities. QUANTIFY is experienced in summarizing the results of this work through meta-analyses and narrative reviews. Our team consists of 50% PhDs and integrated AI solutions to provide our clients with the very best services.
Press
Dynamics of vaccine messages on Twitter

Do you want to know more about the dynamics of #vaccine messages on @Twitter in […]
Much potential in Danish health data
Our country director Mary Rosenzweig has recently co-authored an overview of use of Danish register-data for outcomes research. The article shows the great opportunities of using Danish registries for a wide range of outcomes across therapeutic areas. However, the article also underlines that several available high-quality registers have only been used to a very limited extent which indicates an untapped potential in the use of Danish registers. […]
Looking for an experienced partner to support your evidence generation strategy? Welcome to Quantify!

Last week, ISPOR released their 2022–2023 Top 10 Health Economics and Outcomes Research Trends Report, highlighting high-impact trends in healthcare. These include real world evidence, drug and healthcare pricing, health technology assessment, health data, and artificial intelligence. QUANTIFY is proud to offer experience and expertise in these areas – get in touch with us today to hear more about how we […]
Patients with atopic dermatitis can be accurately identified by algorithm
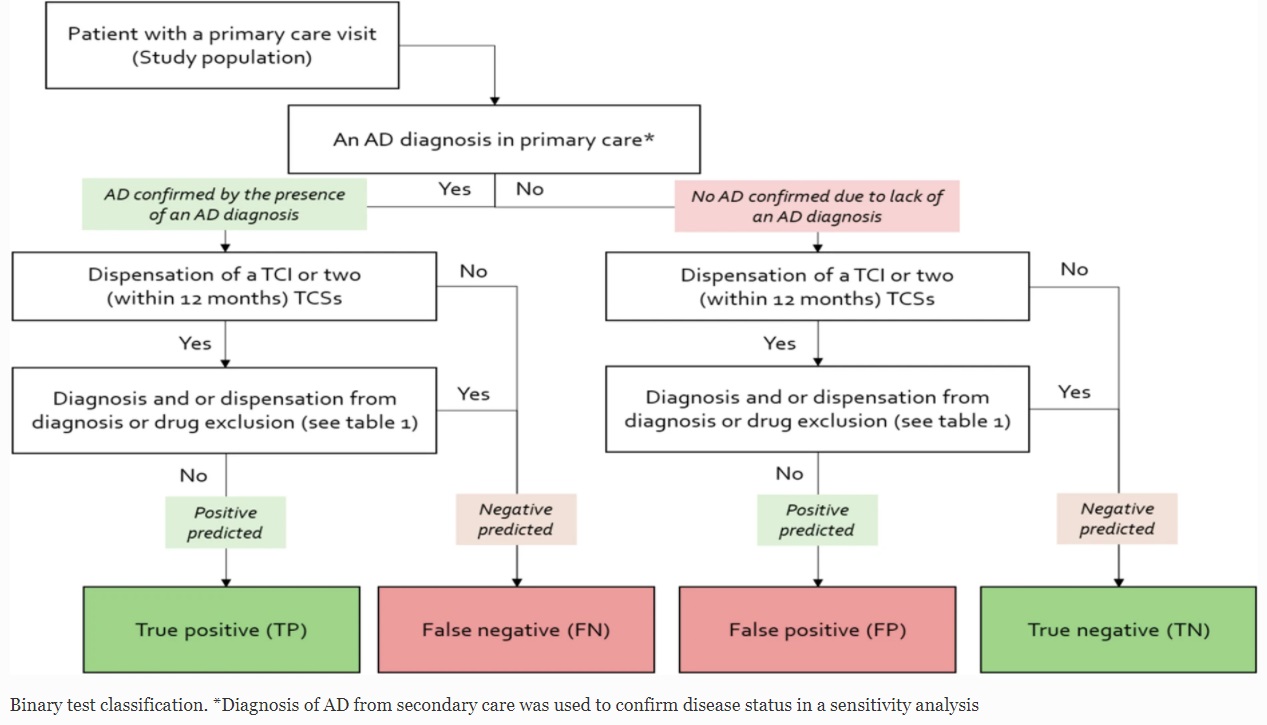 Quantify’s Gustaf Ortsäter, Kirk Geale and Alexander Rieem Dun have published an observational cohort study which validates an algorithm using skin-specific diagnoses from secondary care and pharmacy-dispensed prescriptions to identify patients with atopic dermatitis.
Quantify’s Gustaf Ortsäter, Kirk Geale and Alexander Rieem Dun have published an observational cohort study which validates an algorithm using skin-specific diagnoses from secondary care and pharmacy-dispensed prescriptions to identify patients with atopic dermatitis.
The study shows that pediatric patients with atopic dermatitis can be accurately identified by the algorithm, but further adjustments are required to identify adult patients. This work is important for future studies that […]

