QUANTIFY RESEARCH is a global partner to the pharmaceutical industry in close partnership with academia, patients, public institutions & clinical experts.
Real-world evidence & analytics
Quantify is the leading provider of RWE generated from the world-renouned Nordic data. Through our local presence, expert staff, institutional know-how and more than 10 years’ hands-on experience with the Nordic data, we offer an unparalleled ability to advise and execute Nordic RWE studies with local and global applications. Quantify has a successful track record of advising clients on securing Nordic ethical approval, data access, and optimizing analysis for commercial or regulatory purposes. We also offer RWE services in the EU, UK, and US.
Modelling, Access & Strategy
Quantify is a global provider of health economic evidence and a specialist provider of Nordic market access services including economic models, reimbursement dossiers, and strategic advice. Our experience and expertise ensures an optimized, streamlined market access process across the Nordics. For non-technical stakeholders, Quantify also develops value tools and visualisation dashboards to enhance communication. Our expert staff include ex-payers, ex-pharma, modelling experts, and experienced project managers.
Evidence review & synthesis
Quantify has long standing experience reviewing, interpreting and communicating evidence as part of targeted or systematic literature reviews (SLR). Our team ensures these activities are done systematically, with a focus on usability, to support knowledge management and commercialization activities. QUANTIFY is experienced in summarizing the results of this work through meta-analyses and narrative reviews. Our team consists of 50% PhDs and integrated AI solutions to provide our clients with the very best services.
Press
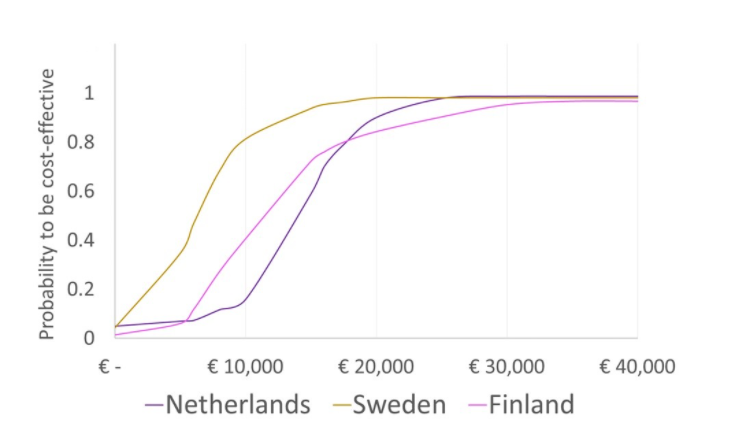
Cost-effectiveness of combination treatment vs monotherapy in COPD
We hope that you did not miss our contribution to the multi-country CEA regarding COPD (Chronic Obstructive Pulmonary Disease) during ISPOR. Click here to see the full poster.
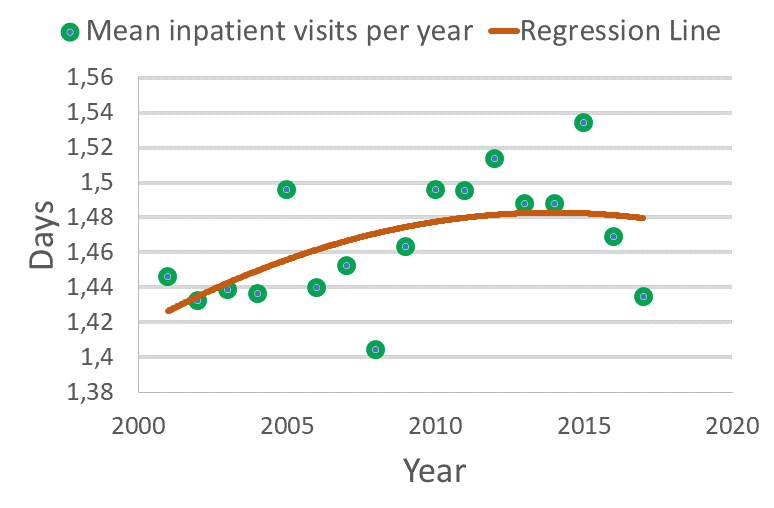
Swedish RWD shows that inpatient care increases in Parkinson’s
We are happy to announce that Quantify’s Trolle Jacobson and Thomas Fast has presented Q-PARK, one of our research platforms at ISPOR. With Q-PARK, it is possible to analyze switches and HCRU for patients with Parkinson´s disease. See the full poster here.
New publication on patient quality of life in Alzheimer’s disease
 A new systematic literature review on patient quality of life in Alzheimer’s disease is now published. The study was co-authored by Anders Gustavsson and is part of the Innovative Medicines Initiative project ROADMAP. The study shows the systematically worse proxy-reported QoL estimates as compared to self-reported estimates across different stages of the disease. The article can be downloaded […]
A new systematic literature review on patient quality of life in Alzheimer’s disease is now published. The study was co-authored by Anders Gustavsson and is part of the Innovative Medicines Initiative project ROADMAP. The study shows the systematically worse proxy-reported QoL estimates as compared to self-reported estimates across different stages of the disease. The article can be downloaded […]
Study of incidence of PsA in PsO co-authored by Quantify published in Acta D-V
Quantify’s Ingrid, Mathias and Kirk are co-authors on an population-based Swedish RWE study of incidence of psoriaticarthritis in adults with skin psoriasis published in Acta D-V, and presented at AAD2020. The results showed that if 10,000 patients with skin psoriasis were followed-up, 169 would be diagnosed with PsA within one year. Risk of psoriatic arthritis was 3.2 times higher amongst patients with severe psoriasis compared with those with mild […]