Overview of Services

Real-world evidence



Health economics and outcomes research



Health policy and health systems analysis



Retrospective studies
Prospective observational studies
Epidemiology and biostatistics



Cost-effectiveness models
Budget impact models
Burden of disease models
Literature reviews
Indirect comparisons and meta-analysis



Disease awareness overviews
Mapping of funding and provision of healthcare
Reimbursement applications



Scientific communication
HEOR value strategy advice
Expert panels and advisory boards
Market Access strategies and tools
Launch optimisation
Web-based interactive value communication apps
We understand healthcare
Our way of work
Identify
What is important, and why: Quantify strives to be a partner which helps clients reach their over-arching goals. During setup of a project, investment is made to understand
a) what is important for the client;
b) what is the background and rationale for the project at hand;
c) who are the relevant stakeholders and what their needs are;
d) are there alternative ways of meeting the objective;
and e) what is the most optimal activity to conduct, and how?
By identifying the framework, and defining a common foundation, we are convinced that we optimize the chance for a successful project.
Throughout a standard project, Quantify’s work to Identify includes mapping out the fundamentals, understanding the stakeholder value perception, defining the evidence gaps and identifying how to translate outcome into value.
Quantify
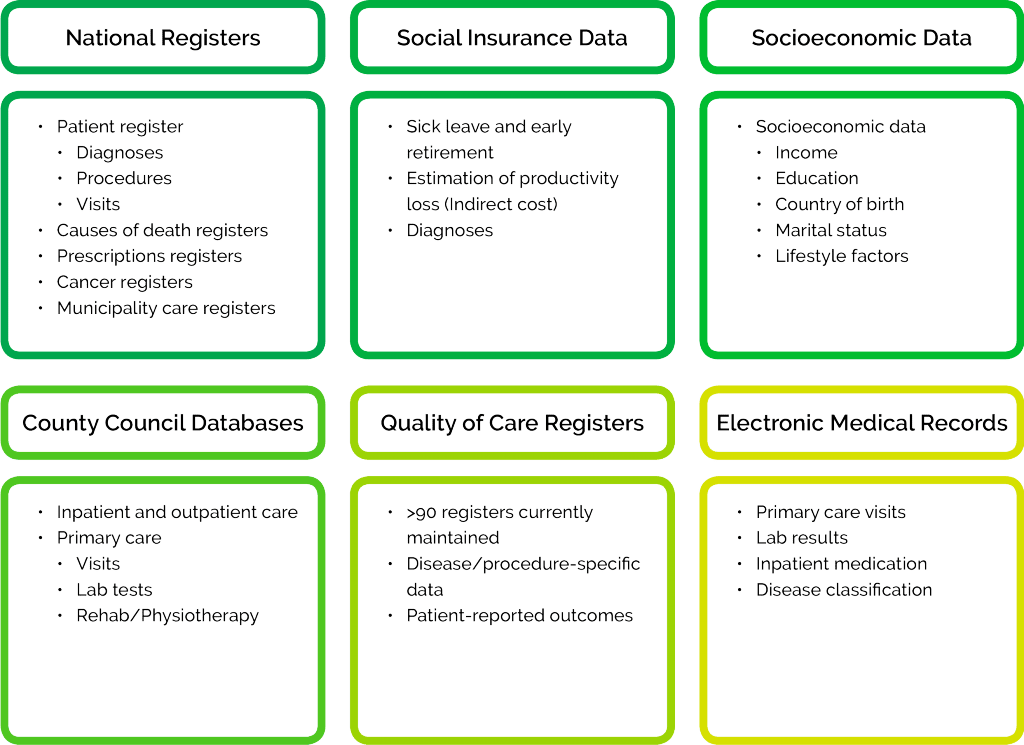
A wide range of methods and data sources can be employed to quantify the value of health care interventions, understand how health care systems operate, and study treatment patterns, epidemiology or patient segments.
Communicate
The communication of study results needs to be carefully considered to ensure that the key messages are presented in a clear and convincing way. Making the complex simple is critical to successfully conveying study findings to the relevant stakeholders.