QUANTIFY RESEARCH is a global partner to the pharmaceutical industry in close partnership with academia, patients, public institutions & clinical experts.
Real-world evidence & analytics
Quantify is the leading provider of RWE generated from the world-renouned Nordic data. Through our local presence, expert staff, institutional know-how and more than 10 years’ hands-on experience with the Nordic data, we offer an unparalleled ability to advise and execute Nordic RWE studies with local and global applications. Quantify has a successful track record of advising clients on securing Nordic ethical approval, data access, and optimizing analysis for commercial or regulatory purposes. We also offer RWE services in the EU, UK, and US.
Modelling, Access & Strategy
Quantify is a global provider of health economic evidence and a specialist provider of Nordic market access services including economic models, reimbursement dossiers, and strategic advice. Our experience and expertise ensures an optimized, streamlined market access process across the Nordics. For non-technical stakeholders, Quantify also develops value tools and visualisation dashboards to enhance communication. Our expert staff include ex-payers, ex-pharma, modelling experts, and experienced project managers.
Evidence review & synthesis
Quantify has long standing experience reviewing, interpreting and communicating evidence as part of targeted or systematic literature reviews (SLR). Our team ensures these activities are done systematically, with a focus on usability, to support knowledge management and commercialization activities. QUANTIFY is experienced in summarizing the results of this work through meta-analyses and narrative reviews. Our team consists of 50% PhDs and integrated AI solutions to provide our clients with the very best services.
Press
✨Thank you for a great evening!✨
Last week, we had the pleasure of hosting a mingle for the life science industry, bringing together colleagues, partners, and new faces for an evening of great conversations and shared inspiration.
A big thank you to everyone who joined us!
Events like these remind us how important it is to create space not just for collaboration, but for connection.
We’re already looking forward to the next one…watch this space! Would you like […]
📢Publication alert!
We are delighted to share that the NORDSTAR manuscript “Use of high-dose inhaled corticosteroids and risk of corticosteroid related adverse events in asthma- findings from the NORDSTAR cohort” is now available online, co-authored by Quantify’s Patrik Sandin, Alexandra Cooper, Olivia Ernstsson, and Kirk Geale, PhD.
NORDSTAR is lead by Celeste Porsbjerg at Bispebjerg Hospital in collaboration with […]
PubMed, Embase and Google Scholar- what´s the difference?
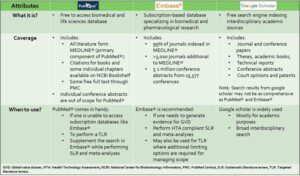
As the volume of literature grows exponentially, regular review and synthesis of evidence from diverse sources is crucial for informed decision-making. Choosing the right database can significantly impact the quality and relevance of your literature search.
Check out this quick reference to help you understand the differences between PubMed, Embase, and Google Scholar, and when to use each.
Do you need to review […]


