QUANTIFY RESEARCH is a global partner to the pharmaceutical industry in close partnership with academia, patients, public institutions & clinical experts.
Real-world evidence & analytics
Quantify is the leading provider of RWE generated from the world-renouned Nordic data. Through our local presence, expert staff, institutional know-how and more than 10 years’ hands-on experience with the Nordic data, we offer an unparalleled ability to advise and execute Nordic RWE studies with local and global applications. Quantify has a successful track record of advising clients on securing Nordic ethical approval, data access, and optimizing analysis for commercial or regulatory purposes. We also offer RWE services in the EU, UK, and US.
Modelling, Access & Strategy
Quantify is a global provider of health economic evidence and a specialist provider of Nordic market access services including economic models, reimbursement dossiers, and strategic advice. Our experience and expertise ensures an optimized, streamlined market access process across the Nordics. For non-technical stakeholders, Quantify also develops value tools and visualisation dashboards to enhance communication. Our expert staff include ex-payers, ex-pharma, modelling experts, and experienced project managers.
Evidence review & synthesis
Quantify has long standing experience reviewing, interpreting and communicating evidence as part of targeted or systematic literature reviews (SLR). Our team ensures these activities are done systematically, with a focus on usability, to support knowledge management and commercialization activities. QUANTIFY is experienced in summarizing the results of this work through meta-analyses and narrative reviews. Our team consists of 50% PhDs and integrated AI solutions to provide our clients with the very best services.
Press
Nordic RWE in FDA decision making
The use of Real-World Data (RWD) to support regulatory decisions is growing rapidly.
Several regulating agencies have in the past few years issued guidelines on the requirements on Real-World Evidence (RWE)-studies to be fit-for purpose for regulatory purposes.
Nordic data properties share many attributes, such as availability of key data elements, high data accuracy and almost universal completeness, highlighted by the recent FDA-guideline as key elements for RWD to be considered […]
COHERE Finland!
On September 17th, Quantify Finland participated in the Council for Choices in Health Care in Finland (COHERE Finland)10th anniversary seminar. The theme of the seminar was “Prioritization and the service portfolio in 2030.”
The day featured several thought-provoking presentations with highlights including:
👉 Perspectives on Finland’s national economy, primary healthcare patients and health centre operations.
👉 Assessment of treatments in Finland is constantly evolving, with an increasing focus on national cost-effectiveness. The event highlighted […]
Quantify annual fall kick-off!🍂

Last Friday Quantify Research‘s Nordic teams came together for the annual fall kick-off at beautiful Hasselbacken in Stockholm.
It was a day packed with interesting presentations, group activities and we even braved the weather for a wet walk along the water in Djurgården.
The weather might’ve dampened our clothes, but it couldn’t rain on our enthusiasm!
Delving into evidence synthesis?
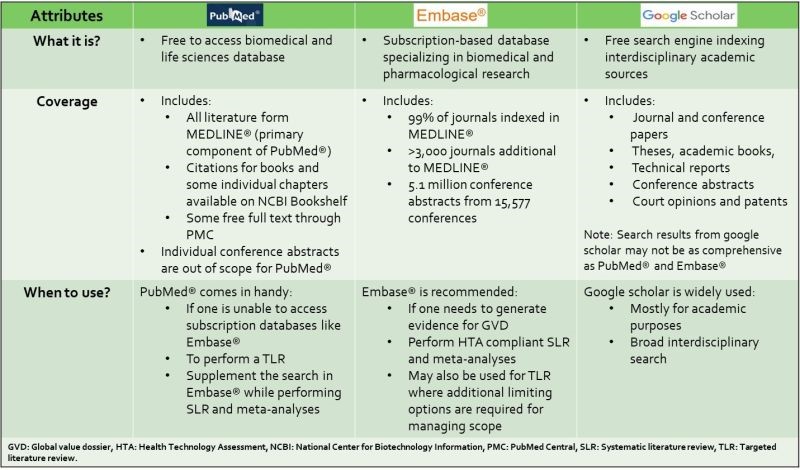
PubMed®, Embase® and Google Scholar – what is the difference and when should I use each?
In the realm of HEOR, synthesizing evidence from diverse sources is crucial for informed decision-making.
Choosing the right database can significantly impact the quality and relevance of your literature search.
Here’s a quick guide to help you understand the differences between PubMed®, Embase®, and Google Scholar, and when to use each.
 […]
[…]

