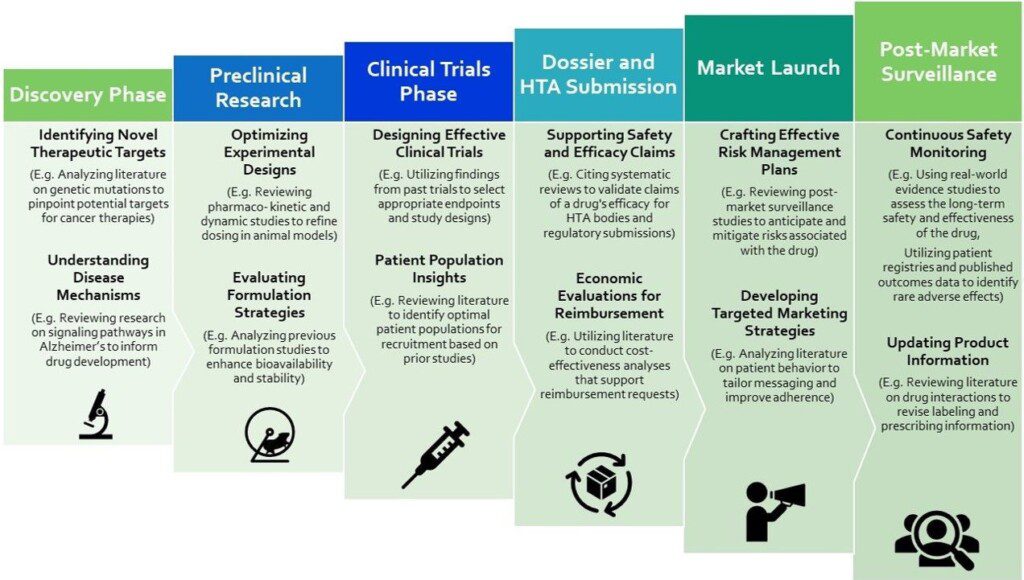
Literature reviews are essential throughout the pharmaceutical product development lifecycle, from identifying therapeutic targets to assisting with regulatory and health technology assessment submissions and ultimately improving drug safety and patient outcomes at every stage.
This image presents the use cases for literature reviews across the life cycle of product development, something our Evidence Review and Synthesis team are experts in!