This week, experts from the Quantify Research Finland group took part in HTA of Medications 2025, hosted by Pharmaca.
It was a day full of rich discussions and valuable insights with top HTA professionals from government and industry.
The perspectives we gained are already feeding into our Market Access & Strategy work, helping us better support our clients navigating the evolving HTA landscape in Finland.
Here are a few key takeaways for those following HTA developments in Finland:
🔹 A shift toward a unified national HTA body: Legal reforms are underway to streamline Finland’s HTA processes. The aim is to establish a single national evaluator, replacing the current fragmented approach where assessments differ based on whether a product is pharmacy- or hospital-dispensed.
🔹 Evolving conditional reimbursement models: While Finland’s economic confidential agreement framework has stabilized, there are ongoing efforts to enhance it—possibly expanding beyond purely price-based schemes to allow greater flexibility and impact.
🔹 Alignment with EU HTA processes: National authorities are already preparing for joint EU HTA evaluations. The first such assessments are expected in 2026, and companies are being guided to proactively provide national PICO input.
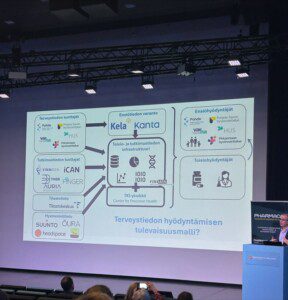
🔹 A national pharmaceutical information database: Is in the making
Finland is developing a centralized database to house key pharmaceutical information including product and reimbursement data. This would mark a major step forward from the current patchwork of systems.
We’re excited to see how these developments shape access to innovative medicines and proud to be part of the conversation.