Earlier this week the U.S. Food and Drug Administration (FDA) granted accelerated approval to Vijoice® (alpelisib) based primarily on real-world evidence (RWE). The decision reflects the increasing importance of real-world data (RWD) for regulatory decision making, alongside recent guidance from the FDA and EMA.
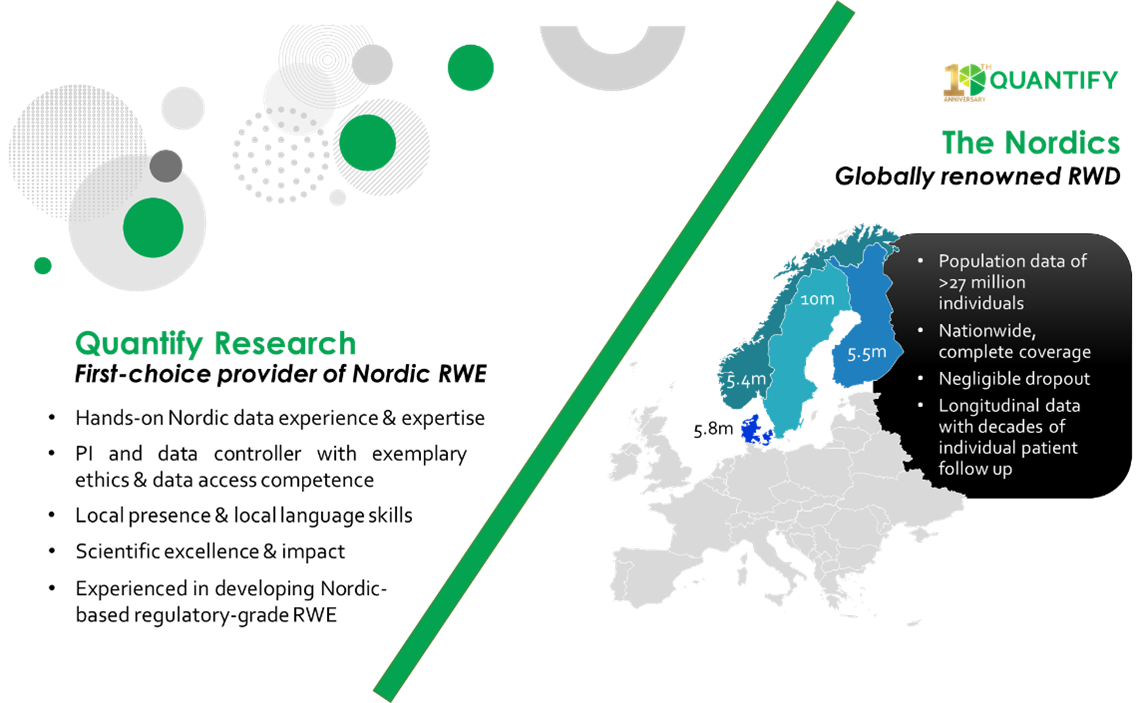
As the leading provider of Nordic RWE studies, with more than 200 executed projects involving RWD, Quantify Research recognizes the critical importance of these data and applauds the recent steps taken by regulatory agencies to do the same. Nordic health data, providing nationwide coverage, linkable medical records and more than 100 disease-specific registries, is uniquely positioned to provide evidence to support regulatory evidence needs and health technology assessments.
Interested to know more? Reach out to info@quantifyresearch.com
#RWD #RWE #realworlddata #realworldevidence #HTA #regulatorygrade #Nordic
