Every year, Quantify Research supports Lif by providing EFPIA – European Federation of Pharmaceutical Industries and Associations with information needed for the W.A.I.T survey for Sweden, and performs a detailed review of national reimbursement of new medicines.
This year’s cohort of the Swedish deep dive includes new medicines with EMA approval in 2020-2022, with an aim to present the advantages and challenges of the system for national reimbursement of medicines – from the perspective of pharmaceutical companies.
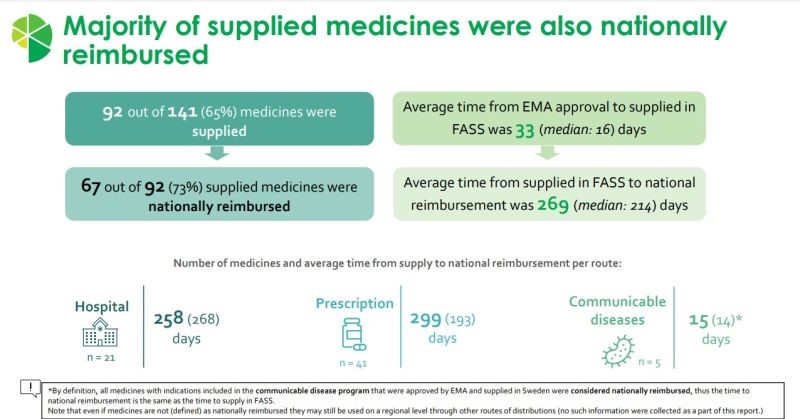
The report highlights that more than two thirds of all supplied medicines in the latest “2020-2022”-cohort are also nationally reimbursed.
Overall, almost half of all new medicines in this cohort are nationally reimbursed.
Hence, some medicines are not supplied nor nationally reimbursed in Sweden. And for others, time to national reimbursement is long.
Possible explanations include:
– Negative national reimbursement decisions (actual or perceived)
– Sweden not being a priority country for the company
– A reimbursement application is under development and/or
– Lack of data, need for price negotiations and other health economic challenges
In addition, many non-reimbursed medicines belong to companies that lack Nordic presence and/or have limited Swedish market experience.
What are your thoughts on potential improvements of the Swedish reimbursement system?
Don’t hesitate to reach out to Quantify’s Konstantin Macheridis or Linnea Oldsberg who worked closely with Karolina Antonov on this report.
The English report can be found here:
and a shorter version in Swedish can be found here: