🎃Halloween Movie Night! 🎃

Spooky season has begun at Quantify Research, which we marked with a scary movie night watching Beetlejuice.
Luckily Diva the guard dog was in the room to […]

Spooky season has begun at Quantify Research, which we marked with a scary movie night watching Beetlejuice.
Luckily Diva the guard dog was in the room to […]
Last week our colleague Nicholas Norton returned from spending some time with the Quantify Research team in Chandigarh. Over the course of two weeks Nicholas managed to squeeze in work, great team building and […]

We are excited to announce our presence at this year’s ISPOREurope in Barcelona!
Over the course of the conference our colleagues look forward to getting some facetime with clients and colleagues and presenting […]
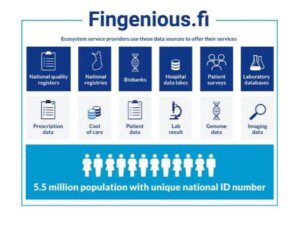
We’re happy to announce that Quantify Research Finland has been accepted to Fingenious® Ecosystem!
The Fingenious® Ecosystem is a collaboration network […]
The landscape of healthcare research is rapidly evolving, and social media is emerging as a valuable resource for real-world evidence (RWE) studies.
In one of Quantify’s publications, 𝐒𝐨𝐜𝐢𝐚𝐥 𝐌𝐞𝐝𝐢𝐚 𝐋𝐢𝐬𝐭𝐞𝐧𝐢𝐧𝐠 𝐟𝐨𝐫 𝐑𝐞𝐚𝐥-𝐖𝐨𝐫𝐥𝐝 𝐄𝐯𝐢𝐝𝐞𝐧𝐜𝐞: 𝐀 𝐍𝐨𝐯𝐞𝐥 𝐀𝐩𝐩𝐫𝐨𝐚𝐜𝐡 𝐭𝐨 𝐇𝐞𝐚𝐫𝐢𝐧𝐠 𝐭𝐡𝐞 𝐏𝐚𝐭𝐢𝐞𝐧𝐭’𝐬 𝐕𝐨𝐢𝐜𝐞, […]


To mark yesterday’s World Mental Health Day the team finished off their workday with a wonderfully relaxing (and sometimes challenging!) yoga session with Devon […]
Literature reviews are essential throughout the pharmaceutical product development lifecycle, from identifying therapeutic targets to assisting with regulatory and health technology assessment submissions and ultimately improving drug safety and patient outcomes at every stage.
This image presents the use cases for […]
Last week, Quantify’s Emilie Toresson Grip was invited to the first Swedish Steatotic Liver Disease (SLD) Think Tank, hosted by Karolinska Institutet.
24 hours packed with speed networking, group discussions, and insightful presentations.
The primary goals? […]

Our CEO Kirk Geale, PhD will be attending the DRUG INFORMATION ASSOCIATION (DIA) Real-World Evidence conference in Philadelphia from October 24-25, 2024!
We are looking forward to meeting with clients and partners to […]
Last week Quantify Research was present at the annual Health Economics Conference (Helseøkonomikonferansen) in Norway.
The conference gathered a broad audience and participation from academia, the healthcare sector, industry, patient organisations and government.
Over two days, […]