New year, new opportunities

Last week, we had the pleasure of hosting several athagoras Group colleagues (Roman-Marcus Rittweger, Charline Camilla Wurzer, and Anton M.) visiting our Stockholm office.
We are […]

Last week, we had the pleasure of hosting several athagoras Group colleagues (Roman-Marcus Rittweger, Charline Camilla Wurzer, and Anton M.) visiting our Stockholm office.
We are […]
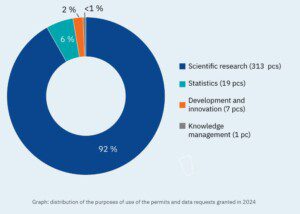
Findata – Finnish Social and Health Data Permit Authority has published its annual review of 2024. Here are some highlights:
🟢 In 2024, Findata turned 5 […]
Last week, we had the pleasure of hosting a mingle for the life science industry, bringing together colleagues, partners, and new faces for an evening of great conversations and shared inspiration.
A big thank you to everyone who joined us!
Events like […]
We are delighted to share that the NORDSTAR manuscript “Use of high-dose inhaled corticosteroids and risk of corticosteroid related adverse events in asthma- findings from the NORDSTAR cohort” is now available online, co-authored by Quantify’s Patrik Sandin, Alexandra Cooper, Olivia […]
Haven’t yet RSVP’d? It’s not too late – click here

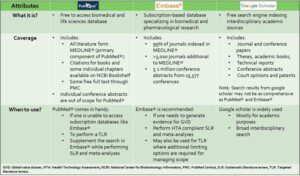
As the volume of literature grows exponentially, regular review and synthesis of evidence from diverse sources is crucial for informed decision-making. Choosing the right database can significantly impact the quality and relevance […]

Here are some of the things that makes our data unique:
1️⃣Fast access to comprehensive data
The average time to fully process applications at The Danish Health Data Authority ( […]
We would like to introduce our latest addition to the Finland team: Olli-Tuomas Kovalainen!
Tuomas holds a research master’s in Economics from the University of Helsinki and has been working with Finnish health data […]

Quantify’s own Béranger Lueza and Abraham Choong are onsite at the World Evidence, Pricing and Access Congress in Amsterdam. The team has spent a busy […]
The decision timelines for Nordic HTA agencies vary depending on the agency and submission process. In Sweden, Tandvårds- och läkemedelsförmånsverket, TLV—responsible for the reimbursement system of outpatient medicines —has a maximum processing time of 180 […]