QUANTIFY RESEARCH is a global partner to the pharmaceutical industry in close partnership with academia, patients, public institutions & clinical experts.
Real-world evidence & analytics
Quantify is the leading provider of RWE generated from the world-renouned Nordic data. Through our local presence, expert staff, institutional know-how and more than 10 years’ hands-on experience with the Nordic data, we offer an unparalleled ability to advise and execute Nordic RWE studies with local and global applications. Quantify has a successful track record of advising clients on securing Nordic ethical approval, data access, and optimizing analysis for commercial or regulatory purposes. We also offer RWE services in the EU, UK, and US.
Modelling, Access & Strategy
Quantify is a global provider of health economic evidence and a specialist provider of Nordic market access services including economic models, reimbursement dossiers, and strategic advice. Our experience and expertise ensures an optimized, streamlined market access process across the Nordics. For non-technical stakeholders, Quantify also develops value tools and visualisation dashboards to enhance communication. Our expert staff include ex-payers, ex-pharma, modelling experts, and experienced project managers.
Evidence review & synthesis
Quantify has long standing experience reviewing, interpreting and communicating evidence as part of targeted or systematic literature reviews (SLR). Our team ensures these activities are done systematically, with a focus on usability, to support knowledge management and commercialization activities. QUANTIFY is experienced in summarizing the results of this work through meta-analyses and narrative reviews. Our team consists of 50% PhDs and integrated AI solutions to provide our clients with the very best services.
Press
Global events may impact our brains
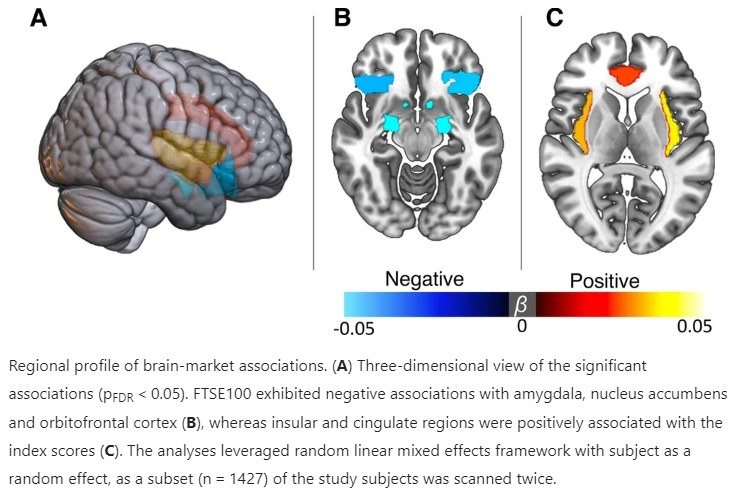
Quantify’s Christoph Abé (PhD) was involved in a large-scale study identifying population-level brain changes related to stock market fluctuations using neuroimaging data from the UK Biobank.
The study, published in Nature: Scientific Reports, showed in about 40,000 individuals that fluctuations in economic indices, such as stock market, are associated with changes in brain structure, mood, and wellbeing on a population-level.
This is of […]
Publication on Care Pathways in Atopic Dermatitis
Want to know more about one of our recent publications? Quantify’s Kirk Geale, Gustaf Ortsäter and Alexander Rieem Dun are co-authors on a recent publication on ”Care Pathways in Atopic Dermatitis”. The study´s objective was to describe longitudinal care pathways including health care management, treatment patterns and disease progression in patients with atopic dermatitis. The study found that healthcare contacts and use of atopic dermatitis indicated […]
Priyanka Saini joins Quantify!

Today we finally get to welcome Priyanka Saini who will strengthen our administrative team as Admin / HR Manager at our office in Chandigarh. With 8+ years of experience within HR, Administration, Marketing & Operations, she will be a strong addition to the team.
Thank you for choosing Quantify, Priyanka!
Tine Kopp joins Quantify as Senior Lead Analyst!

Today we are delighted to introduce Tine Iskov Kopp who recently joined our Danish team as Senior Lead Analyst.
Tine has a PhD in molecular epidemiology and extensive experience with designing and conducting epidemiological studies encompassing clinical data, genetic data, questionnaires, and administrative health register data. After completion of her PhD, Tine has worked in the healthcare industry, providing epidemiological consultancy […]

