In the world of market access, a large amount of resources go into engineering all-singing, all-dancing global models with a clean look and friendly user interface while simultaneously providing value to local affiliates. Although this feat is certainly an accomplishment, it is important to remember the importance of model quality control (QC). This activity is a core component of model development and reduces the risk of errors that can lead to biased results. In the case of global model development, a serious error can affect patient access across many local markets.
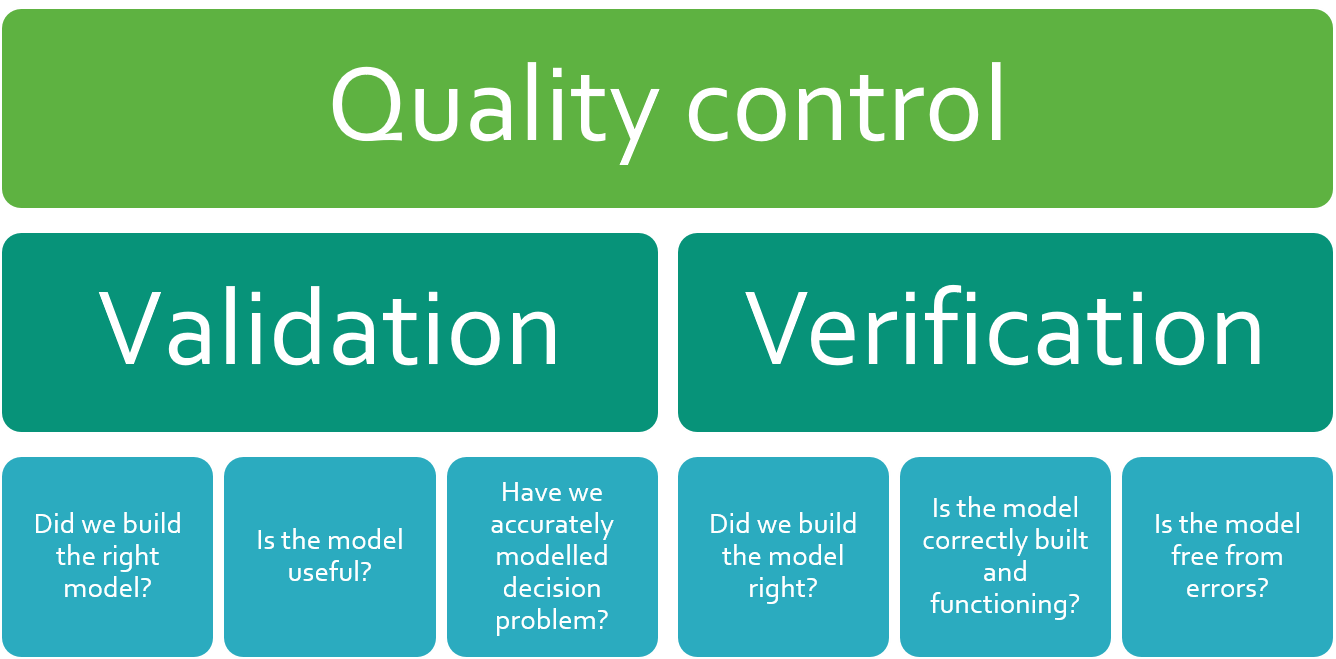
Taking the approach used in software development, QC can be divided into validation and verification (1). Validation evaluates whether the model meets the requirements of decision makers. For example, validation asks if the model includes the right patient population and subgroups, if the relevant comparators included, and if the model’s structure (e.g. Markov) is appropriate. Verification, on the other hand, focuses on whether the model is working as it should and is free from errors. In this case, a modeler might test that the results show zero costs if all unit costs are set to zero, or all navigation buttons take the user to the correct sheet.
Quantify has an in-house QC process, combining aspects of validation and verification in three levels: basic, extended, and full quality control. These represent increasing levels of reliability, and clients can select the desired level based on the needs of any given project.
The QC process is vital to both manufacturers and assessors, and the task should not fall by the wayside. Speak with our consultants to learn more about the Quantify approach to quality control.
Short summary:
Quality control is an essential part of model development. This exercise is often divided into model validation and verification, both of which indirectly promote patient access and give assurance to health technology assessors. Quantify has an in-house quality control process that helps to ensure the reliability of economic models.
(1) Validation and verification may be referred to using other terminology, such as internal and external validity.
Suggested further reading:
Kleijnen, Jack P.C.. Theory and Methodology, Verification and validation of simulation models. European Journal of Operational Research 82 (1995) 145-162 http://faculty.kfupm.edu.sa/ics/salah/misc/courses/ics533/slides/Kleijnen1995.pdf.
Sculpher M, Fenwick E, Claxton K. Assessing Quality in Decision Analytic Cost-Effectiveness Models, A Suggested Framework and Example of Application. PharmacoEconomics May 2000, Volume 17, Issue 5, pp 461–477 https://link.springer.com/article/10.2165/00019053-200017050-00005
Checklist in Annex 9.1 in Methods for the Economic Evaluation of Health Care Programmes, Fourth Edition (2015). Oxford University Press. Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW.
